|
The genome-wide association study
of the Alzheimer’s Disease Genetics Consortium A collaborative effortThe Alzheimer's Disease Genetics Consortium (ADGC) Genome-wide Association Study (GWAS) was launched as a collaborative effort, leveraging the collective resources of the AD research community, to identify Alzheimer’s disease genes. ADGC, led by Gerard Schellenberg at the University of Pennsylvania, is a partnership involving:
ADGC seeks to identify genetic variability that influences susceptibility to AD. Susceptibility genes potentially influence age of onset and rate of progression through the prodromal and mild cognitive impairment (MCI) phases of the disease. In addition, ADGC looks for genes that influence specific AD-related endophenotypes, such as neuropathology features (e.g., amyloid load, tangle load), biomarker measures (e.g., Aß and tau levels in cerebrospinal fluid, MRI measures), rate of disease progression, and responses to environmental factors (e.g., drugs, non-pharmaceutical environmental factors).
NACC's roleNACC maintains a large database of standardized data — the Uniform Data Set (UDS) — collected by the ADCs. NACC cleans and curates the data to ensure they are as complete as possible and makes them available for research. For the GWAS project, NACC provides each ADC with a list of its own subjects that are eligible for the GWAS, tracks which samples have been submitted for DNA banking, and then notifies the ADGC and ADCs of the amount owed for the cost of sending the samples. (See reimbursement flow chart.) NCRAD sends DNA out for genotyping; once the genotyping is complete, those data are sent to ADGC. Finally, NACC sends HIPAA research-limited phenotypic data to ADGC, so that the phenotypic data can then be paired with the genotype data. The GWAS collaborative effort diagram illustrates the data and sample submission process. 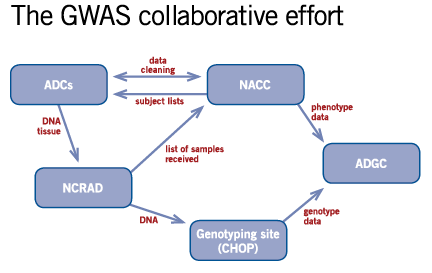
Reimbursement 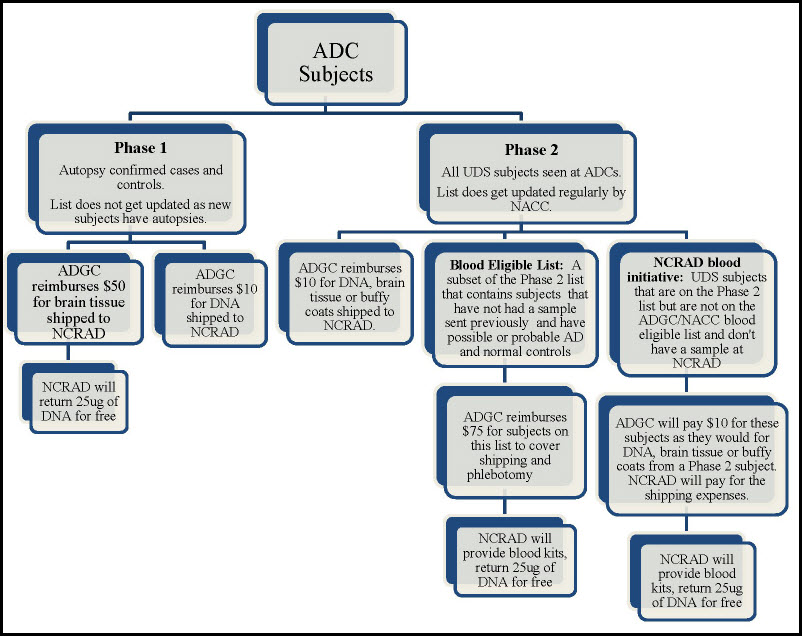
|